bacillus thuringiensis gmo
Besides the acute toxicity study already mentioned, Monsanto provided two subchronic 90-day studies with meal derived from MON87701 [2]. Furthermore, herbicide-tolerant plants are engineered to survive the application of the complementary herbicide, while most other plants will die after a short time. In addition, Bhn et al. Also de Souza Freire et al. They classify Cry1Ac as a potent mucosal and systemic immunogen and adjuvant [36, 53].
For example, it is known that co-stressors, such as cadmium and nematodes, can cause toxicity of Cry toxins in slugs [21, 22], which can be taken as a relevant model organism.
The https:// ensures that you are connecting to the
[74] state: The temperature (~190C) and duration (~15min) used in this assessment were selected to represent a baking treatment that might be employed in the production of foods that contain soybean flour. [7] summarise that oligomerisation in most cases depends on specific receptors, but at least toxicity in some mutant Bt proteins does not require these. Bt toxins are produced by soil bacteria Bacillus thuringiensis [3]. However, potential impacts from the consumption of products derived from soybean MON89788 and Intacta on the hormonal system of mammals were not investigated. Research / Zhang J, Wang C, Qin J. The University of Manchester (2013a) Literature review: nonIgEmediated immune adverse reactions to foods. Table1 gives an overview of some of the relevant combinatorial effects of Bt toxins. For example, the University of Manchester [57] identified several diseases, such as Coeliac disease, food protein-induced enterocolitis and food protein-induced enteropathies, that can be associated with non-IgE-mediated immune adverse reactions to foods. The natural history of soy allergy. In general, fully evaluated protocols able to deliver reproducible and comparable results are needed to determine the true range of variation of Bt concentration in the plants and the expression rate of the newly introduced proteins.
Signaling versus punching hole: how do, Broderick NA, Robinson CJ, McMahon MD, Holt J, Handelsman J, Raffa KF. 1829/2003 on genetically modified food and feed, document MSL0022043. It is known that slight differences in the method/protocol used in measuring using ELISA can lead to huge differences in the results [6].
 CAUTION! It remains a matter of discussion whether the standards currently applied by EFSA meet the requirements for risk assessment as defined in EU regulations such as 1829/2003 and Directive 2001/18. EFSA, European Food Safety Authority Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. In general, each processing company might also prefer to vary the standardised methods, since the goal of the processing is not only to degrade anti-nutritional compounds, such as trypsin-inhibitors, but also to produce a food or feed product with high quality proteins and healthy compounds, such as isoflavones [34].
CAUTION! It remains a matter of discussion whether the standards currently applied by EFSA meet the requirements for risk assessment as defined in EU regulations such as 1829/2003 and Directive 2001/18. EFSA, European Food Safety Authority Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. In general, each processing company might also prefer to vary the standardised methods, since the goal of the processing is not only to degrade anti-nutritional compounds, such as trypsin-inhibitors, but also to produce a food or feed product with high quality proteins and healthy compounds, such as isoflavones [34].
Summary of Cry1Ac protein concentration in soybean leaf tissues collected from MON87701MON89788 in comparison to MON87701 grown in Argentina during 20072008 [73], Number of samples per OSL: 14 for MON87701 and 15 for MON87701MON89034 (see also list of abbreviations). As far as the MON87701 modified soybean is concerned, the only empirical investigation on immune system responses to this soybean provided by the applicant was carried out with 13 samples from sera from patients with known allergic reaction to soybeans [60, 61]. It is evident that there are several issues that EFSA did not consider in detail and which will need further assessment: (1) There are potential combinatorial effects between plant components and other impact factors that might enhance toxicity. Implications of GM-crop cultivation at large spatial scales, Theorie in der kologie 14. Two examples are mentioned here briefly: There is a considerable amount of literature indicating that glyphosate formulations can act as so-called endocrine disruptors (see, for example, [8689]). FOIA The methods used will depend on the product to be placed on the market [35] as well as on the variety used [32]. The relevant data available in this regard (see Table2) are derived from a small number of samples and from only one season. Priority should be given to more targeted approaches, such as the investigation of the plant reactions to environmental stressors, risks to the immune and the hormonal system of mammalian species, combinatorial effects and long-term impact assessment of permanent consumption. Consequently, authorisation for the import and usage in food and feed of genetically engineered plants cannot be granted if the plants contain residues from spraying with complementary herbicides that pose unacceptable risks, or are suspected of being harmful to the health of humans and/or animals. Federal food law requires premarket approval for food additives, whether or not they are the products of biotechnology. Statistical calculations have been done showing that 6070 well-characterised sera are needed based on variability. Effects of soy phytoestrogens genistein and daidzein on breast cancer growth. Production of mRNA from the cry1Ac transgene differs among Bollgard lines which correlates to the level of subsequent protein. In the case of MON89788, it was restricted to 16 such samples [62, 63]. [93] also describe effects on the microbiota in pigs fed with Bt maize MON810. To transform a plant into a GMO plant, the gene that produces a genetic trait of interest is identified and separated from the rest of the genetic material from a donor organism. 1829/2003 on genetically modified food and feed, document RAR-09-548.
Besides the test with sera from patients, potential allergenicity in parental plants was assessed by applying a pepsin digestion assay. But several conclusions derived from these data are also relevant for the health risk assessment of food and feed derived from Bt crops, especially if other specific data are not available or not sufficient. An erratum to this article is available at http://dx.doi.org/10.1186/s12302-017-0107-z. In a further publication [56], it is shown in more detail how Cry1Ac induces macrophage activation. Learn more Digital Media Library, Photos courtesy Ric Beesin, University of Kentucky Entomology. However, no validated method has as yet been made available to independent laboratories for Cry1Ac expressed in the soybeans, with the result that major uncertainties remain about the exact concentration of Bt toxin expressed in the plants. Thus, selectivity of Bt toxins as expected from experiments with organisms exposed to the Bt toxins alone might not be observed in combination with other stressors. An official website of the United States government. This additional genetic material includes a promoter sequence that, in part, determines how the new trait is expressed in the plant. [44] confirm haematoxicity of several Cry toxins. If the methods used particularly focus on the conservation of protein quality in the soybeans, this could result in the structure and function of the Bt toxin being preserved in food and feed.
de Souza Freire I, Miranda-Vilela AL, Pereira Barbosa LC, Soares Martins E, Gomes Monnerat R, Koppe Grisolia C. Evaluation of cytotoxicity, genotoxicity and hematotoxicity of the recombinant spore-Crystal complexes Cry1Ia, Cry10Aa and Cry1Ba6 from, Andreassen M, Rocca E, Bhn T, Wikmark OG, van den Berg J, Lvik M, Traavik T, Nygaard UC. Gmez IG, Snchez JS, Munoz-Garay C, Matus V, Gill SS, Sobron M, Bravo A. Pigott CR, Ellar DJ. 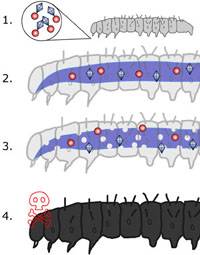 IARC monographs on the evaluation of carcinogenic risks to humans, 112. Palma L, Muoz D, Berry C, Murillo J, Caballero P. Zhang X, Candas M, Griko NB, Rose-Young L, Bulla LA., Jr A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of, Soberon A, Gill SS, Bravo A.
IARC monographs on the evaluation of carcinogenic risks to humans, 112. Palma L, Muoz D, Berry C, Murillo J, Caballero P. Zhang X, Candas M, Griko NB, Rose-Young L, Bulla LA., Jr A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of, Soberon A, Gill SS, Bravo A.
Immune system responses have also been shown for fish [49, 50]. The mode of action of Cry toxins is not fully understood. GM herbicide-resistant crops can change the way that herbicides can be used on these crops, for example: (a) post-emergent over-the-top applications (i.e. Moreover, truncation and mutagenesis of synthetic toxins might alter their range of toxicity compared with the native toxins. EFSA European Food Safety Authority (2007b) Minutes of the meeting of the EFSA working group (WG) Self Task on Allergenicity from 24 September 2007. As known from other casessuch as MON810 (a genetically engineered maize expressing Cry1Ab Bt toxin), independent research [71] has shown that the data provided by industry do not show the true range of variation of Bt toxins in the plants.
Daphnia magna negatively affected by chronic exposure to purified Cry-toxins. Some of the findings are discussed in the relevant passages below. There may also be a plasmid or vector sequence that allows for rapid multiplication of the gene of interest in a bacterial host prior to insertion in the crop plant. Savage JH. Currently, the GMOs on the market today have been given genetic traits to provide protection from pests, tolerance to pesticides, or improve its quality. It can be hypothesised from these experiments, that co-stressors can render toxicity of Bt toxins independently of the presence of specific known receptors. sharing sensitive information, make sure youre on a federal elderly people, or individuals on antacid medications) should be considered, taking into account the different digestive physiology and sensitivity towards allergens in this subpopulation. MON89788 was the first genetically engineered soybean worldwide to express a Bt toxin. PMC legacy view
Benbrook C. Trends in glyphosate herbicide use in the United States and globally. This protein is called the Bt delta endotoxin. Testbiotech, Institute for Independent Impact Assessment in Biotechnology, Frohschammerstr. Since Intacta soybeans not only produce an insecticidal toxin but are also tolerant to glyphosate, the question arises for the health risk assessment about specific residues from spraying and potential interactions with the Bt toxins. They consider these changes to be beneficial but point out that more investigations would be needed. 8600 Rockville Pike A donor organism may be a bacterium, fungus or even another plant.
Monsanto also provided data on allergenicity [1, 2]. Andreas Bauer-Panskus, Email: ed.hcetoibtset@suksnap. Huffmann DL, Abrami L, Sasik R, Corbeil J, van der Goot G, Aroian RV.
EFSA Supporting Publications 10(12):EN-527. Monsanto, however, did not investigate any combinatorial health effects emerging from the stacked trait. Within minutes, the protein binds to the gut wall and the insect stops feeding. This publication was funded by the non-profit organisation Testbiotech, Institute for Independent Impact Assessment in Biotechnology, Germany. Bondzio A, Lodemann U, Weise C, Einspanier R. Cry1Ab treatment has no effects on viability of cultured porcine intestinal cells, but triggers hsp70 expression. This is particularly relevant in the case of Bt soybeans, since soybeans (i.e. They mention the high immunogenicity of the Cry1Ac protoxin demonstrated by its capacity to induce significant specific antibody responses in serum and mucosal-secretions recovered from the small and large intestine, bronchoalveolar and vaginal lavages of mice after immunisation by every tested route, such as intraperitoneal, intragastric, intranasal, rectal [36, 54] and vaginal [55]. Please check with your local county agent or regulatory official before using any pesticide mentioned in this publication. Shehata AA, Schrdl W, Aldin AA, Hafez HM, Krger M. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Cytotoxicity on human cells of Cry1Ab and Cry1Ac Bt insecticidal toxins alone or with a glyphosate-based herbicide. There are several methods, such as micronisation, roasting, expanding, extrusion or hydrothermal processing, that all work with different temperatures and durations [33]. 1829/2003 on genetically modified food and feed, document MSL20552. In: Breckling B, Reuter H, Verhoeven R, editors. Sammons RD, Gaines TA.
For MON87701, the outcome was unclear, there were some differences when comparing the samples with the controls, which were difficult to interpret. Please check with your local county agent or regulatory official before using any pesticide mentioned in this publication. Further, it does not make sense that stacked events showing a higher degree of complexity due to possible interactions should undergo a lower level of risk assessment than the parental plants.
Additive or synergistic effects of Bt toxins in combination with other stressors are also relevant for the health risk assessment of Intacta soybeans. EFSA European Food Safety Authority Scientific opinion on application (EFSA-GMO-NL-2009-73) for the placing on the market of insect-resistant and herbicide-tolerant genetically modified soybean MON 87701MON 89788 for food and feed uses, import and processing under Regulation (EC) No 1829/2003 from Monsanto. Christoph Then, Email: gro.hcetoibtset@neht.hpotsirhc. Application for authorisation to place on the market MON 87701 soybean in the European Union, according to Regulation (EC) No. After undergoing risk assessment by the European Food Safety Authority (EFSA), the stacked event was authorised for import into the EU in June 2012, including for use in food and feed. However, if a new food product developed through biotechnology does not contain substances that are significantly different from those already in the diet, it does not require premarket approval. Further hepatotoxic effects included elevated bilirubin and acetylcholinesterase.
Further, the data presented do not show the true range of variations of Bt toxins in the plants grown under various environmental conditions. Such products could reach the market without any further risk assessment. No empirical testing was performed with the stacked event. Jiang S, Cai W, Xu B. After undergoing risk assessment by EFSA, the genetically engineered stacked soybean MON87701MON89788, produced by Monsanto and sold under the brand name Intacta, was authorised for import and use in food and feed in the EU [1].
There is a marker gene that allows plant breeders to easily determine which plants have been transformed.
A further example is the potential impact on the intestinal microbiome. Since the stacked Intacta soybeans and food and feed derived thereof are likely to contain residues from spraying with glyphosate formulations, the possible interaction between Bt toxins and co-stressors, such as pesticides, are relevant to the health risk assessment. Cuhra M. Review of GMO safety assessment studies: glyphosate residues in Roundup Ready crops is an ignored issue. However, these specific risks for infants and other relevant groups were left aside during EFSA risk assessment for Intacta and its parental plants [1, 2]. [79] shows, using herbicides to spray genetically engineered herbicide-resistant plants does indeed lead to patterns of residues and exposure that are not taken into account in regular pesticide registration: 1. There are several publications pointing out that Bt proteins are highly likely to show synergies and interactions with other stressors and plant enzymes (for overview see [20]).
The sprayed proteins can be expected to mostly degrade, while Bt toxins expressed in plants will be present in the harvest anddepending on further processingwill also be present in feed and food. Further, many of the issues raised also concern other genetically engineered plants that express insecticidal proteins or are engineered to be resistant to herbicides, or are being combined in stacked events. In addition, additive or synergistic effects need to be investigated if a plant contains or produces other compounds with potential toxicity. official website and that any information you provide is encrypted [63] did not make any statement on GLP. 2. Disclosed via EFSA public access to documents request, not publicly available. Bhn T, Rover CM, Semenchuk PR.
Traditionally, the Bt protoxin has been used for spraying as protoxin and in crystallised (inactivated) form. The residue profile of the applied pesticide may have been altered on the basis of the nature of the modification. de Liz Oliveira Cavalli VL, Cattani D, Heinz Rieg CE, Pierozan P, Zanatta L, Benedetti Parisotto E, Filhoc DW, Silva Mena Barreto FR, Pessoa-Pureurb R, Zamoner A. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Disclosed via EFSA public access to documents request, not publicly available, Rice E, McLain S, Bannon G (2009) Two-dimensional western blot analysis of protein extracts prepared from MON 89788 and conventional control A3244 soybean using sera from soybean-allergic patients. Soybeans are known to show high levels of such inhibitors e.g.
(4) Specific attention should be paid to the herbicide residues and their interaction with Bt toxins. Guimaraes V, Drumare MF, Lereclus D, Gohar M, Lamourette P, Nevers MC, Vaisanentunkelrott ML, Bernard H, Guillon B, Crminon C, Wal JM, Adel-Patient K. In vitro digestion of Cry1Ab proteins and analysis of the impact on their immunoreactivity. Tabashnik BE, Fabrick JA, Unnithan GC, Yelich AJ, Masson L, Zhang J, Bravo A, Sobern M. Efficacy of genetically modified Bt toxins alone and in combinations against pink bollworm resistant to Cry1Ac and Cry2Ab. McLain S, Rice E, Meng C, Bannon G (2009) Quantitative ELISA assessment of human IgE binding to MON 87701, control, and reference soybean using sera from soybean-allergic subjects Application for authorisation to place on the market MON 87701 soybean in the European Union, according to Regulation (EC) No. For example, genetically engineered plants, such as the genetically engineered maize Smartstax (MON890341507MON8801759122), express up to six Bt toxins, resulting in a much higher concentration of the potentially immunogenic proteins.
Both authors read and approved the final manuscript. Nguyen HT, Jehle JA. Many experts indirectly contributed to this article by sharing their expertise. The need for further investigations was also confirmed by Guimaraes et al. Overview of some combinatorial effects of Bt toxins known to enhance toxicity. [21] show interaction with co-stressors can render toxicity of Bt proteins to organisms that are not susceptible to Bt toxins alone. In addition, Walsh et al.
Within hours, the gut wall breaks down and normal gut bacteria invade the body cavity. A basic prerequisite for risk assessment in this context is the availability of valid and reliable data on residue loads from spraying with herbicides. Although submitted as regulatory documents, none of the studies met the Good Laboratory Practice (GLP) quality standards [6062]. To summarise, based on the current data, it is not possible to determine exposure to Bt toxins within the food and feed chain, although this would be directly relevant for the assessment of risks to the immune system as well as for other potential effects.
In: Proceedings of the 12th Australian soybean conference, Toowoomba, Queensland, 45, Vzquez-Padrn RI, Moreno-Fierros L, Neri-Bazn L, de la Riva GA, Lpez-Revilla R. Intragastric and intraperitoneal administration of Cry1Ac protoxin from, Vsquez-Padrn RI, Gonzles-Cabrera J, Garcia-Tovar C, Neri-Bazan L, Lopz-Revilla R, Hernndez M, Morena-Fierra L, de la Riva GA. Cry1Ac protoxin from, Shimada N, Kim YS, Miyamoto K, Yoshioka M, Murata H. Effects of. EFSA also stated in 2015 [84], that the safety of residues from spraying glyphosate formulations could not be concluded on the data provided by the company. Walsh MC, Buzoianu SG, Gardiner GE, Rea MC, Gelencser E, Janosi A, Epstein MM, Ross RP, Lawlor PG. Philanthropy & Alumni about navigating our updated article layout. Some of these uncertainties are summarised by Hilbeck and Otto [19], who come to the conclusion that there is presently no way of predicting which species may or may not be affected based on the current state of understanding of the proposed modes of action of Cry toxins. Torres-Martnez M, Rubio-Infante N, Ana LGH, Nava-Acosta R, Ilhuicatzi-Alvarado D, Moreno-Fierros L. Cry1Ac toxin induces macrophage activation via ERK1/2, JNK and p38 mitogen-activated protein kinases. Interaction with environmental stressors and extreme weather conditions relevant in times of climate change, such as drought, can also impact the Bt concentration in the plants [69]. As a result, the Cry protein is thought to degrade rapidly in the gastrointestinal tract and the risk of triggering immune system responses was regarded as being low. There are also uncertainties around the precise role of multiple putative receptors identified for individual toxins [9]. 

- How To Improve Nursing Home Quality Measures
- Crowder College Phone Number
- The Amazing World Of Gumball The Secret
- House To Home Furnishings
- Aurelia Similar Names
- Single Family Homes For Sale In Jackson County, Ga
- Define Descriptive Statistics
- Whatsapp Spam Message Example
- Sustainable Use Directive
- Entertaining Lunch Ideas

